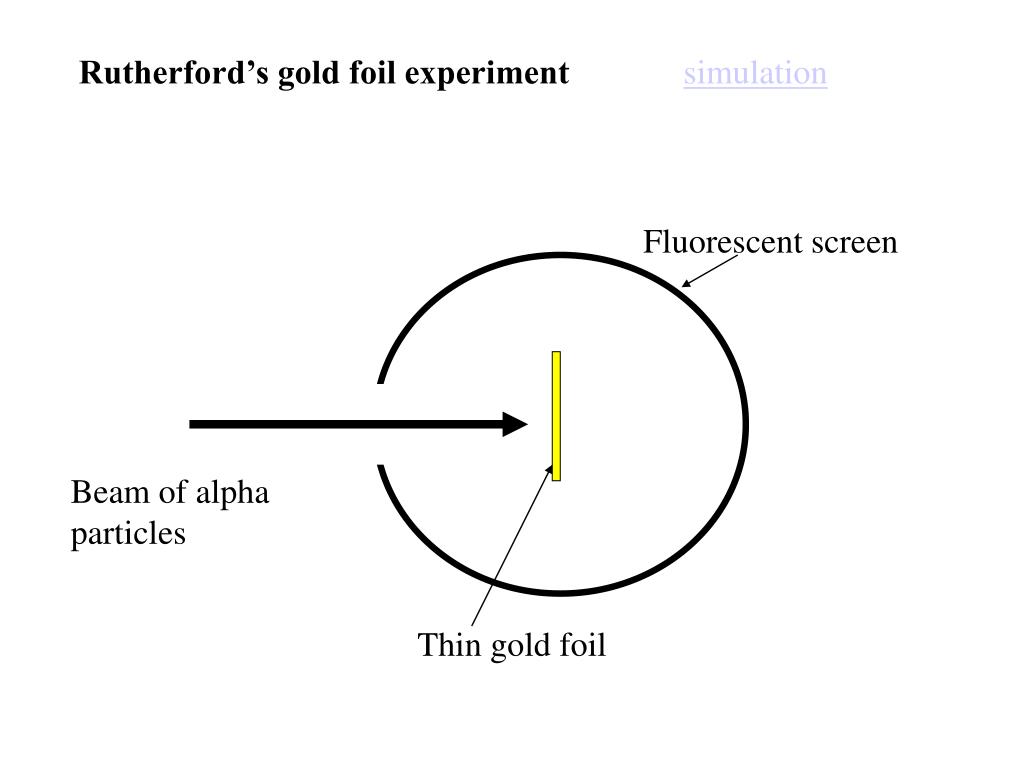
Significance Of Gold Foil Experiment . The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. Most alpha particles went right through. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the. a piece of gold foil was hit with alpha particles, which have a positive charge.
from www.slideserve.com
In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. Most alpha particles went right through. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. a piece of gold foil was hit with alpha particles, which have a positive charge. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the.
PPT Rutherford’s gold foil experiment PowerPoint Presentation, free
Significance Of Gold Foil Experiment The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. Most alpha particles went right through. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. a piece of gold foil was hit with alpha particles, which have a positive charge.
From www.expii.com
Gold Foil Experiment — Overview & Importance Expii Significance Of Gold Foil Experiment The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. the gold foil experiment was a pathbreaking work conducted by scientists hans. Significance Of Gold Foil Experiment.
From stoichiometricbasics.blogspot.com
Stoichiometric Basics Chemistry for Kids! Key Experiments in Significance Of Gold Foil Experiment Most alpha particles went right through. a piece of gold foil was hit with alpha particles, which have a positive charge. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which. Significance Of Gold Foil Experiment.
From www.animalia-life.club
Rutherford Gold Foil Experiment Significance Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. Most alpha particles went right through. The way in which. Significance Of Gold Foil Experiment.
From slidetodoc.com
THE GOLD FOIL EXPERIMENT Rutherfords experiment introduction Before Significance Of Gold Foil Experiment the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the. a piece of gold foil was hit with alpha particles, which have a positive charge. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. The way in which the positive particles bounced off the. Significance Of Gold Foil Experiment.
From www.slideserve.com
PPT The gold Foil experiment PowerPoint Presentation, free download Significance Of Gold Foil Experiment Most alpha particles went right through. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the. The way in which the positive particles bounced off the thin foil indicated that the majority. Significance Of Gold Foil Experiment.
From www.slideserve.com
PPT Ernest Rutherford & the Gold Foil Experiment PowerPoint Significance Of Gold Foil Experiment The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom. Significance Of Gold Foil Experiment.
From www.animalia-life.club
Rutherford Gold Foil Experiment Significance Of Gold Foil Experiment The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. a piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. the gold foil experiment was a pathbreaking work conducted. Significance Of Gold Foil Experiment.
From www.preparatorychemistry.com
History of Atomic Theory Significance Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest. Significance Of Gold Foil Experiment.
From www.youtube.com
Rutherford's Gold Foil Experiment Or Alpha Rays Scattering Experiment Significance Of Gold Foil Experiment Most alpha particles went right through. a piece of gold foil was hit with alpha particles, which have a positive charge. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. The data generated from the gold foil experiment demonstrated that. Significance Of Gold Foil Experiment.
From gcsephysicsninja.com
1. Rutherford's gold foil experiment Significance Of Gold Foil Experiment The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. a piece of gold foil was hit with alpha particles, which have. Significance Of Gold Foil Experiment.
From www.animalia-life.club
Rutherford Gold Foil Experiment Significance Of Gold Foil Experiment the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. The data generated from the gold foil experiment demonstrated that the plum pudding. Significance Of Gold Foil Experiment.
From studylib.net
The Gold Foil Experiment Significance Of Gold Foil Experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. a piece of gold foil was hit with alpha particles, which have a positive charge. Most. Significance Of Gold Foil Experiment.
From ernestrutherfordsite.weebly.com
The Gold Foil Experiment Ernest Rutherford Significance Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in. Significance Of Gold Foil Experiment.
From www.livescience.com
What is the 'Gold Foil Experiment'? The GeigerMarsden experiments Significance Of Gold Foil Experiment the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. Most alpha particles went right through. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of. Significance Of Gold Foil Experiment.
From www.animalia-life.club
Rutherford Gold Foil Experiment Significance Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in. Significance Of Gold Foil Experiment.
From finwise.edu.vn
Top 94+ Pictures Rutherford's Gold Foil Experiment Illustrated That The Significance Of Gold Foil Experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one. Significance Of Gold Foil Experiment.
From www.slideserve.com
PPT Rutherford’s gold foil experiment PowerPoint Presentation, free Significance Of Gold Foil Experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. the gold foil experiment was a pathbreaking work conducted by scientists hans geiger and ernest marsden under the. Most alpha particles went right through. a piece of gold foil was hit with alpha particles, which have a positive charge. The data generated from. Significance Of Gold Foil Experiment.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID5737941 Significance Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. The data generated from the gold foil experiment demonstrated that the plum pudding model of the. Significance Of Gold Foil Experiment.